101
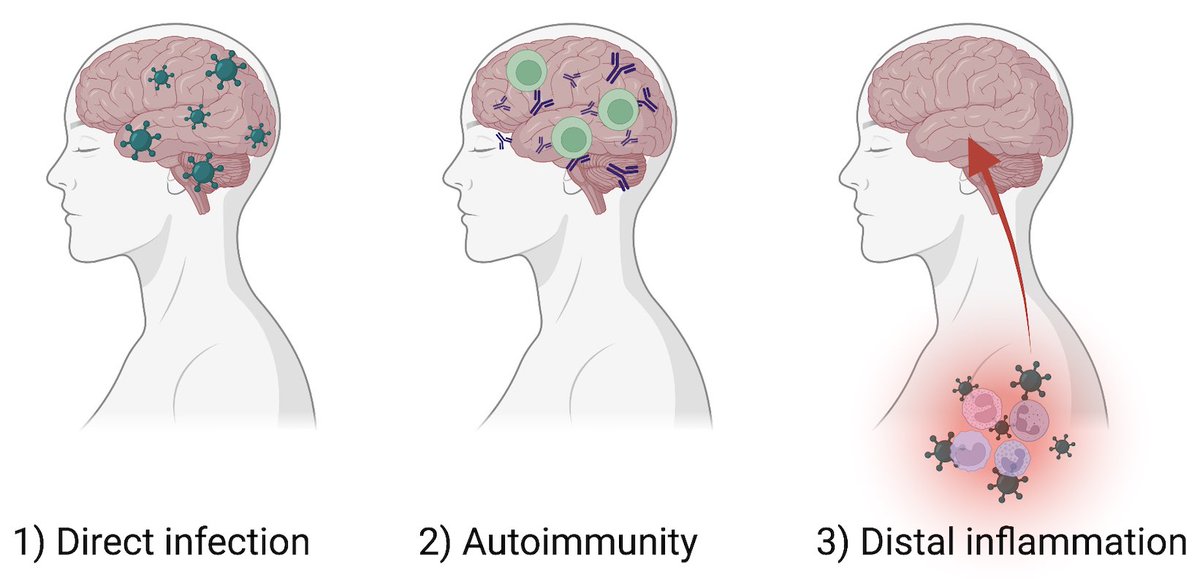
In a nutshell, this study illustrates that respiratory-only mild SARS-CoV-2 infection can lead to detrimental changes in the brain, likely mediated by inflammatory factors. Similar neuropathobiology may be shared in chemo-brain, post-ICU syndrome and ME/CFS. (15/)
102
This study was led and executed by my amazing colleague, @michelle_monje & her team + @PutrinoLab @MountSinaiNYC, @nathavindra et al. From our Yale team, I wish to highlight @peowenlu @ericsongg and others listed here 💪🏼 (14/)
103
104
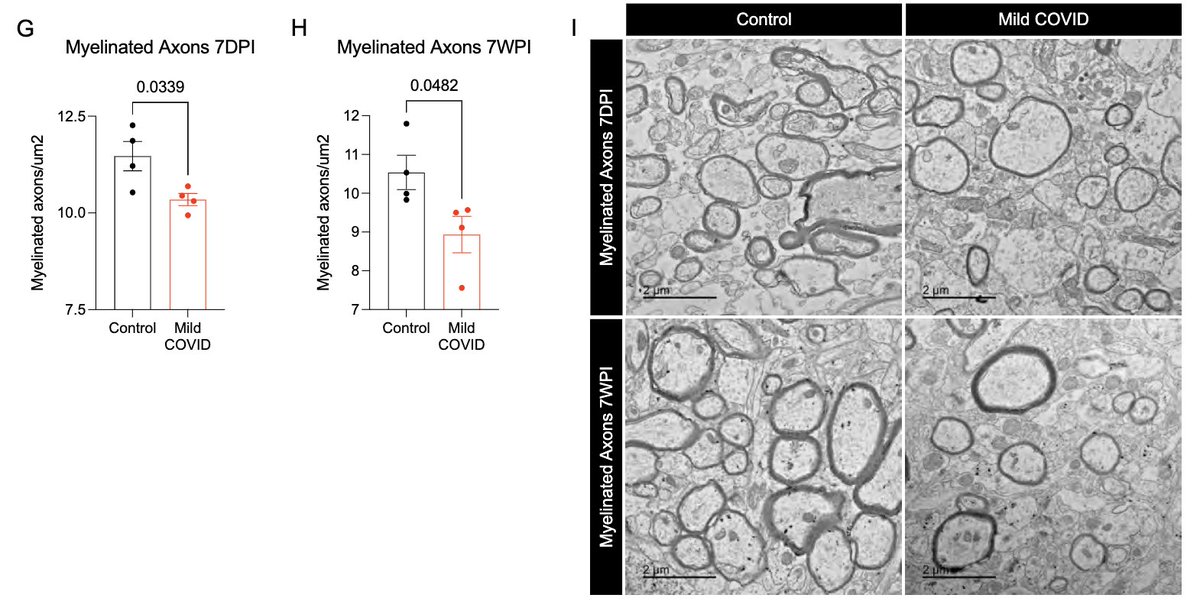
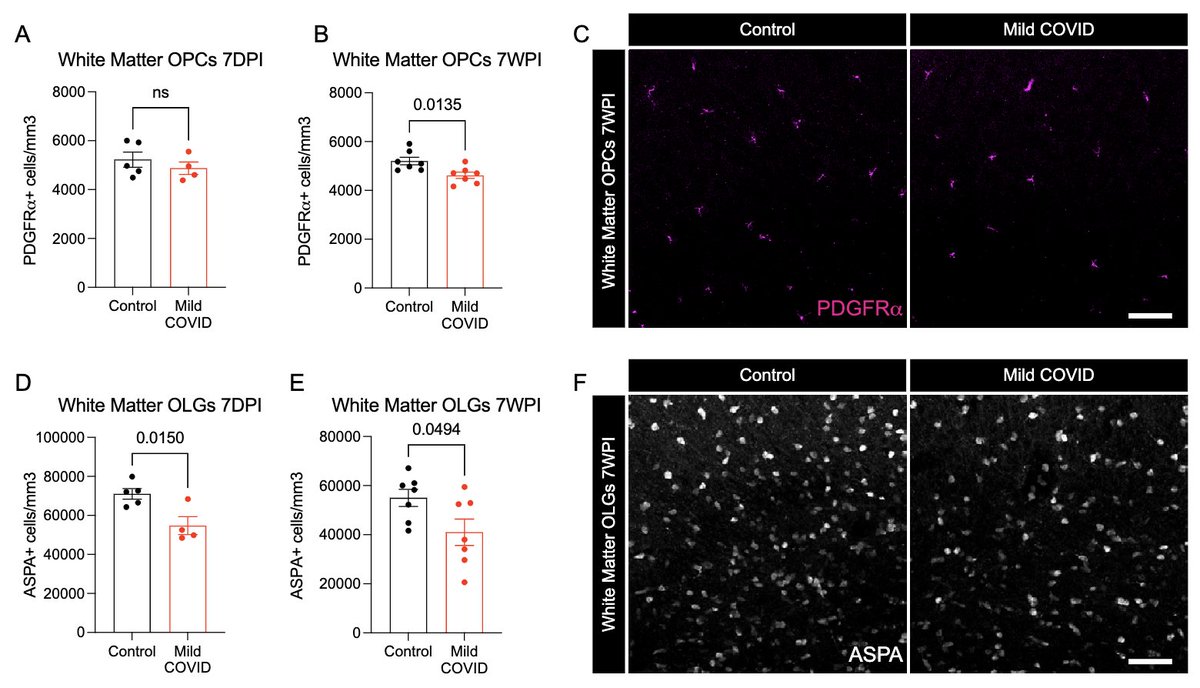
What other changes are happening in the brain of mice with mild respiratory infection? Within just 7 days of infection, we found a loss of ~1/3 of oligodendrocytes, which persisted for at least 7 weeks! Analysis by @ThisIsAnthonyFC and @AnnaGeraghty2 (12/)
105
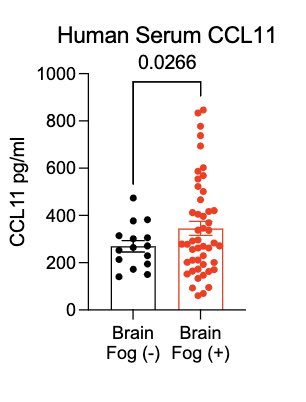
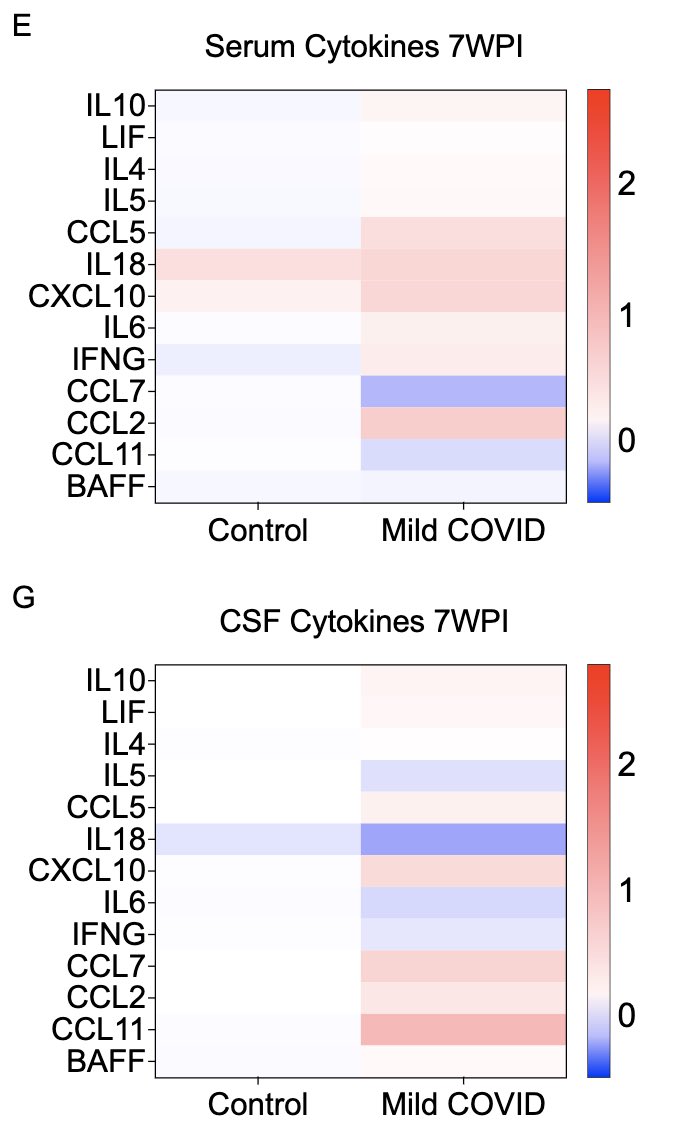
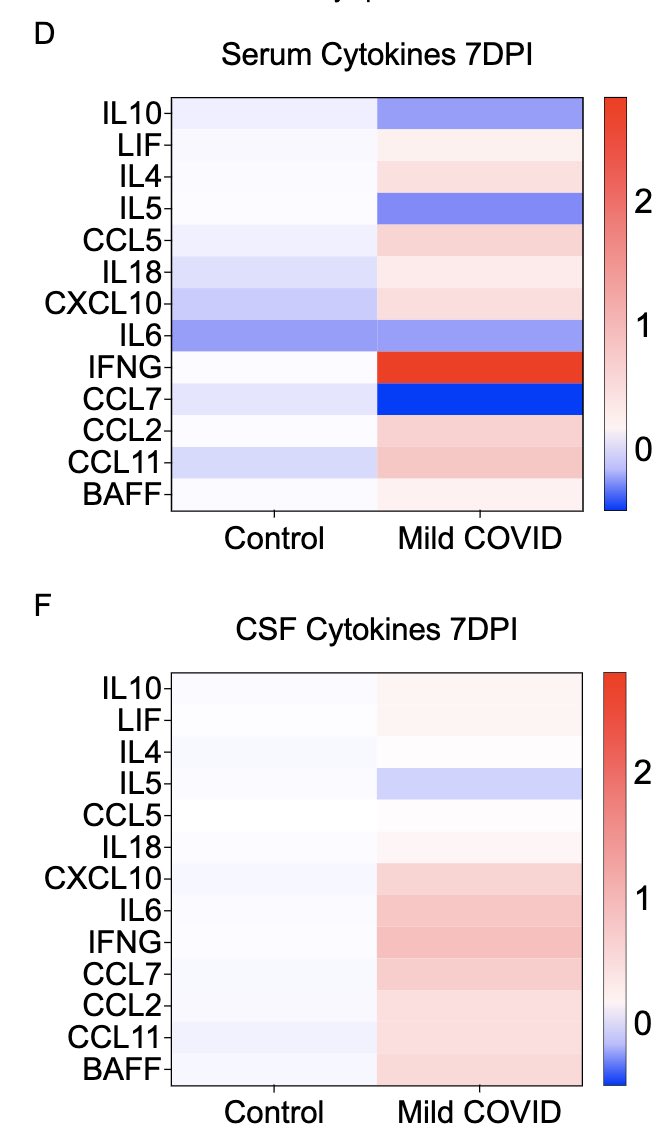
In collaboration with @PutrinoLab at @MountSinaiNYC, we found significantly elevated circulating levels of CCL11 in long COVID patients who reported brain fog vs. those who did not. Many 🙏🏼 to @wood_jamie_1 @LauraTabacof @GeneticHeartDoc #DaynaMcCarthy & the patients! (11/)
106
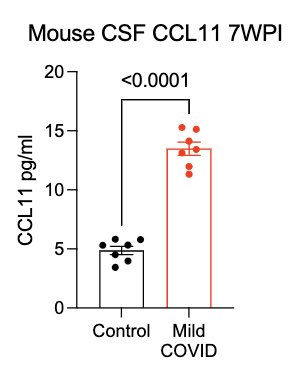
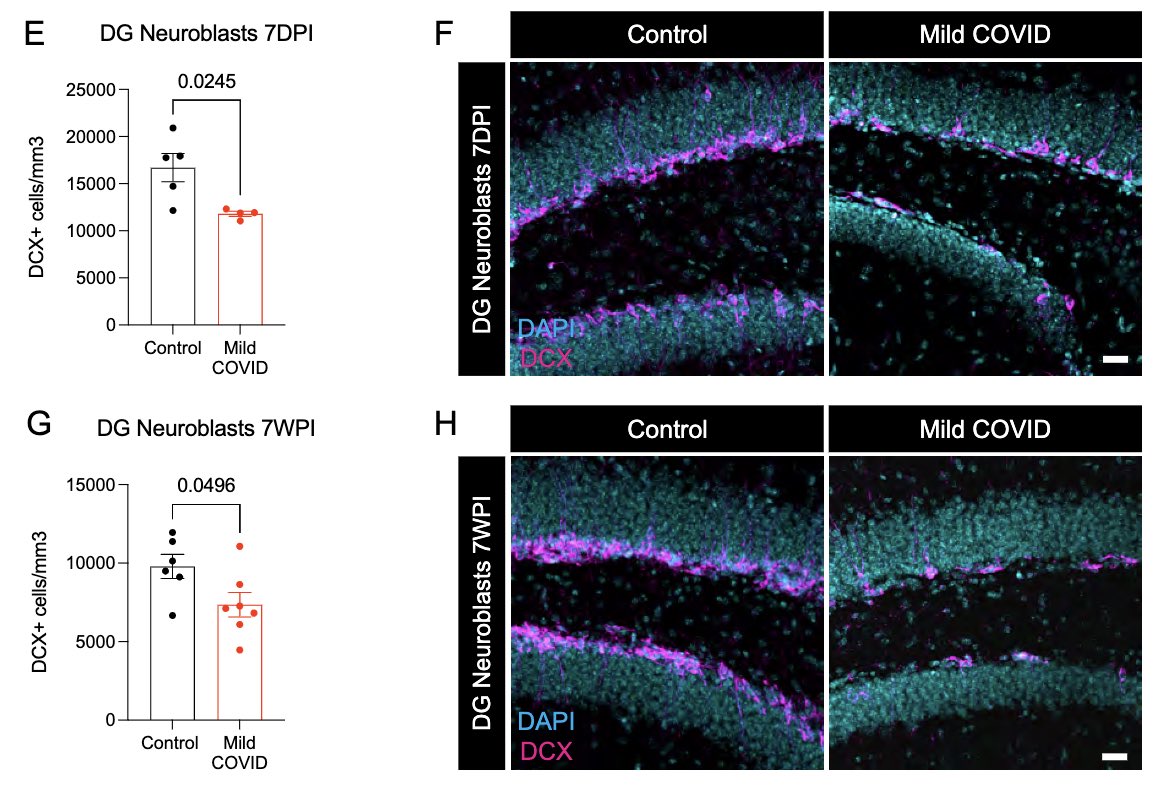
What can lead to impaired neurogenesis in hippocampus? We looked into a chemokine called CCL11 (eotaxin-1) which was shown to reduce neurogenesis (Villeda et al). In our mice, CCL11 was elevated in the CSF 7 weeks after mild respiratory infection. (10/)
pubmed.ncbi.nlm.nih.gov/21886162/
107
108
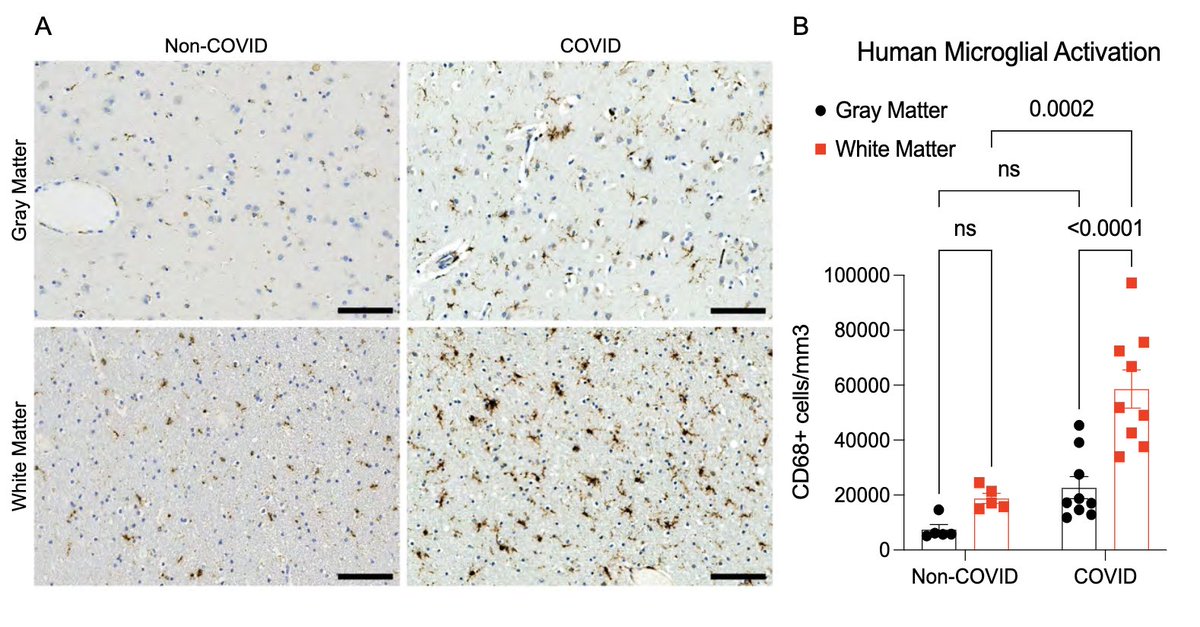
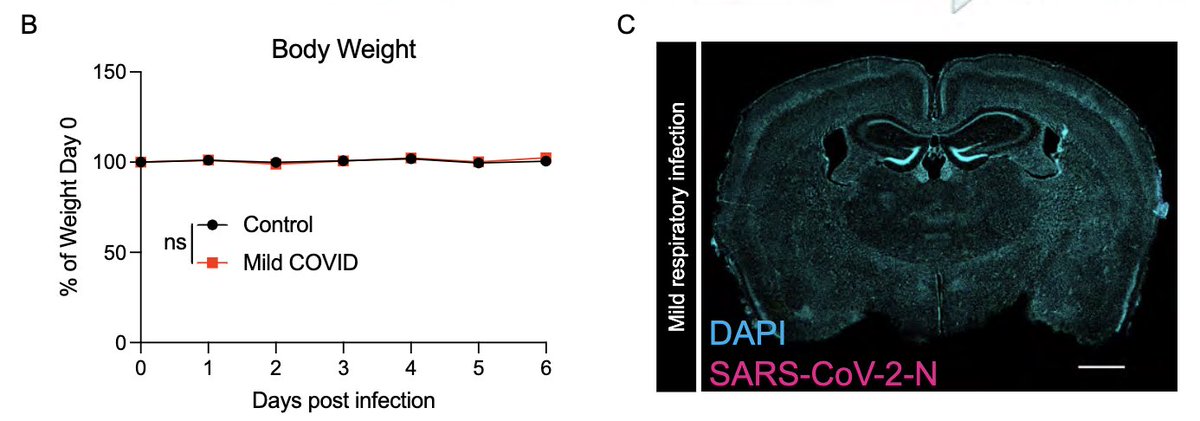
Next, with @nathavindra, autopsies from 9 individuals found to be SARS-CoV-2-positive by nasal swab PCR at the time of death were examined. Brains from those with even mild or asymptomatic SARS-CoV-2 infection had microglial reactivity in subcortical white matter. (8/)
109
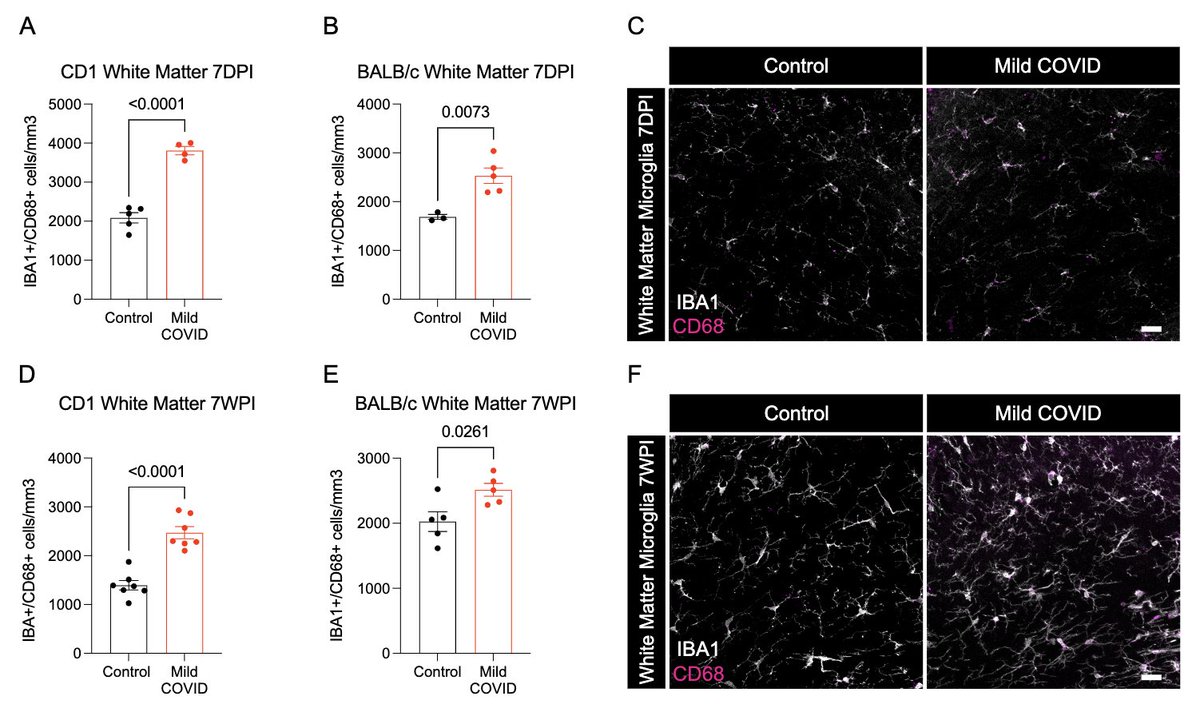
What does a respiratory-only mild COVID do to the brain? @ThisIsAnthonyFC and @AnnaGeraghty2 examined the subcortical white matter of two independent strains of mice and found consistently increased microglial reactivity at 7 days and 7 weeks post infection. (7/)
110
111
112
113
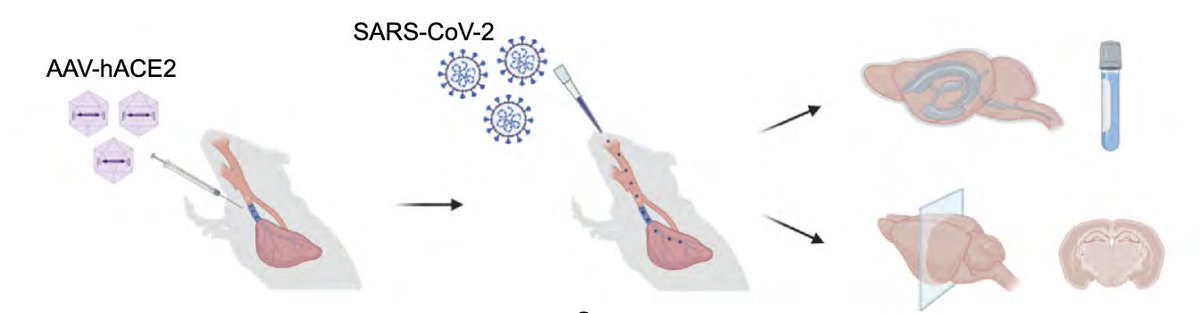
To achieve this goal, @peowenlu & @ericsongg used a mouse model developed by @BenIsraelow & @ericsongg in which we can control where the infection happens. Using AAV-hACE2 intratracheally, we can confine the SARS-CoV-2 infection only to the lungs. (3/)
rupress.org/jem/article/21…
114
115
So excited to be a part of this important study led by @michelle_monje on how significant longterm neurologic damage can occur after a mild respiratory-only SARS-CoV-2 infection. My own🧵on the findings of this study with relevance to #longCovid (1/)
biorxiv.org/content/10.110…
116
Has anyone else with #longCOVID received monoclonal antibodies to SARS-CoV-2? Please share your experience. More data will help justify a clinical trial. Thank you. twitter.com/AlisaValdesRod…
117
118
119
120
CoronaVac is an inactivated SARS-CoV-2 vaccine approved for use in 48 countries. In collaboration with the Ministry of Health in Dominican Republic, we tested whether CoronaVac (2x) + Pfizer booster induces neutralizing Abs to Delta and Omicron. (1/)
medrxiv.org/content/10.110…
121
In #longCOVID patients, “exercise capacity was primarily limited by impaired systemic EO2 of such severity that what should have been an adequate increase in DO2 was insufficient to allow for an increase in VO2.” Via @YalePCCSM
sciencedirect.com/science/articl…
122
So the problem in #longCOVID is not necessarily O2 supply (thus normal cardiopulmonary function) but O2 extraction and thus consumption by tissue. What could cause such defects? Microvascular abnormality? I would love expert input here. @KaminskiMed, @CharleszYaleMed?
123
This thread is about our new preprint on transposable element called long interspersed nuclear elements (LINE-1/L1). When expressed excessively in the cerebellum, L1 causes ataxia (impaired coordination).
A fascinating finding by @taka_takehiro 👇🏽 (1/)
biorxiv.org/content/10.110…
124
These results indicate that nasal vaccines induce IgA and promote better cross-protective immunity against viral variants, and suggest its utility in combating COVID-19 variants of concern. A great write up by Bill Hathaway. (13/)
news.yale.edu/2021/12/10/nas…
125
Our new study by @JieunOh9 @ericsongg @MiyuMoriyama et al shows that immune priming via intranasal route provides superior protection against heterologous respiratory virus challenge. The key is in inducing local secretory IgA with broader coverage. (1/)
science.org/doi/10.1126/sc…